The process is called melting.

We think you are located in United States. Is this correct?
We use this information to present the correct curriculum and to personalise content to better meet the needs of our users.
|
Previous
Chapter 5: Atoms
|
Next
Chapter 7: Chemical reactions
|
Chapter overview
5 weeks
This chapter builds on the introduction to the arrangement of particles in materials that was covered in the chapter 'Solids, Liquids and Gases' of the Gr. 6 Matter and Materials curriculum. In Gr. 6, no distinction was made between atoms and molecules. These were grouped together and the generic term 'particle' was used to refer to these fundamental building blocks of matter. This was the first introduction to the concept of matter particles. The behaviour of particles in each of the three different states of matter was used to explain the macroscopic properties of each state. In this chapter these ideas are further expanded, using the particle model of matter. Important links are made to new concepts such as diffusion, changes of state, density, expansion, contraction and gas pressure. The particle model of matter will be a strong theme throughout the rest of the Physical Sciences curriculum, especially if learners continue through to Gr. 10-12.
2.1: What is the particle model of matter? (1 hour)
|
Tasks |
Skills |
Recommendation |
|
Activity: Changes of state revision |
Accessing and recalling information, revising |
Suggested (revision) |
2.2 Solids, liquids and gases (3 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Comparing solids, liquids and gases |
Accessing and recalling information, comparing |
CAPS suggested |
|
Investigation: Comparing the diffusion of particles in a gas and in a liquid |
Hypothesising, observing, identifying variables, recording information, comparing, interpreting information |
CAPS suggested |
2.3 Change of state (2 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Changes of state |
Crossword puzzle, reading and writing, sorting and classifying |
Optional revision |
|
Investigation: What happens when we heat and then cool candle wax? |
Predicting, hypothesising, planning investigation, drawing and labelling, observing, recording, analysing information |
CAPS suggested |
|
Activity: Hot water balloon |
Observing, recording information |
Optional extension |
2.4 Density, mass and volume (1 hour)
|
Tasks |
Skills |
Recommendation |
|
Activity: Which material is more dense? |
Doing investigation, observing, comparing, communicating and group discussion |
CAPS suggested |
2.5 Density and states of matter (1 hour)
|
Tasks |
Skills |
Recommendation |
|
Activity: Which has the highest density: a solid, a liquid or a gas? |
Comparing, interpreting |
Suggested |
2.6 Density of different materials (3 hours)
|
Tasks |
Skills |
Recommendation |
|
Investigation: Comparing the densities of sand, flour, water and air |
Hypothesising, identifying variables, planning investigation, doing investigation, observing, recording information, interpreting information |
CAPS suggested |
|
Activity: Rainbow density column |
Demonstrating densities, comparing, observing, drawing, comparing |
Suggested |
|
Activity: Some density calculations |
Problem-solving, calculations |
Optional extension |
2.7 Expansion and contraction of materials (2 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: How much longer? |
Drawing graphs, interpreting information, predicting, demonstrating |
CAPS suggested |
|
Activity: How does a thermometer work? |
Revision, comparing, identifying |
Suggested |
2.8 Pressure (2 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Understanding gas pressure |
Following instructions, observing, interpreting information |
CAPS suggested |
Can you remember learning that matter can exist in three different states? What are the three states called?
Solids, liquids and gases.
Can you remember the properties of the different states of matter? Discuss this in your class. Look at the following diagram of the states of matter to help you. Remember to take some notes as you discuss in class.
Get learners to discuss this briefly in small groups and draw a table on the board to summarise learners' ideas. Out of the class, three groups could be chosen randomly, and each group could say what they know about one of the states. Some of the properties that learners should already be familiar with are listed in the following table:
|
Gases |
Liquids |
Solids |
|
|
|

In this chapter we are going to review what we know about solids, liquids and gases. We are going to learn about a scientific model that can be used to describe how the particles in all three states behave. This model is called the particle model of matter and it will help us understand much more about the properties of solids, liquids and gases. Let's get started!
In the previous chapter we learnt that scientists use models when they want to describe things that are difficult to understand. We discussed a model of the atom that helped us to imagine what atoms look like.

Theories are similar to models. They explain scientific phenomena (things and events that can be described and explained in scientific terms) using pictures and words.
The particle model describes matter in a very specific way. It describes four important aspects of matter:
This links back to Gr. 6 Energy and Change where the topics of stored energy and movement energy were covered. In Term 3, Energy and Change, these concepts will be defined more formally as kinetic energy (movement energy) and potential energy (stored energy).
It is very important to note the misconception here that there is 'air' in between the particles. This is NOT true. The spaces between the particles are empty - called a vacuum. Take note to make sure you do not introduce this misconception.
If you need to, turn back to chapter 1 to revise the terms atom, element, compound and molecule and how they relate.
The particle model of matter is one of the most useful scientific models because it describes matter in all three states. Understanding how the particles of matter behave is vital if we hope to understand science!
The model also helps us to understand what happens to the particles when matter changes from one state to another.
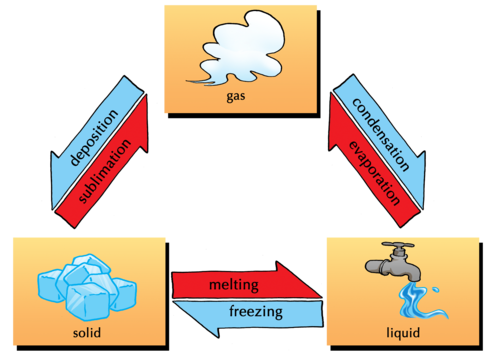
The following diagram shows different changes of state, as well as which processes are the reverse of each other. Melting and freezing are the reverse processes of each other and so are evaporation (boiling) and condensation.
Under special circumstances, a solid can change directly into a gas without melting first. This process is known assublimation and its reverse (when a gas changes directly into a solid without condensing first) is called deposition.
INSTRUCTIONS:
QUESTIONS:
The process is called melting.
Freezing.
We can put it in a warm spot, or heat it in some other way.
It must first melt to become a liquid and then evaporate to become a gas.
Condensation.
Heating is adding energy.
They vibrate or move faster.
This is because they now have more kinetic energy. This introduces the next topic and how we explain the changes of state using the particle model of matter.
We will use the model to look at each of these changes more closely. But first, we will look at how the model describes each state of matter.
We can use the particle model to help us understand the behaviour of each of the states of matter. We are going to look at each state in turn.
There is one very important thing to remember when we consider the different states of matter. For any matter, the individual particles of that matter are exactly the same in all three states, solid, liquid and gas. It is the behaviour of the particles that changes in each state.
This video shows us the different ways that particles behave in the solid, liquid and gaseous states.
Solids keep their shape and cannot be compressed. Let us see if the particle model can help us understand why solids behave in this way.
In a solid, the particles are packed close to each other in fixed positions. They are locked into place, and this explains why solids have a fixed shape. Look at the following images of sodium chloride (table salt). Do you remember the formula for sodium chloride?
Sodium chloride is NaCl. Ask learners why they think the chloride atoms are the bigger purple atoms and the sodium atoms are the smaller yellow ones in the submicroscopic view in the table. The colour does not make a difference, as long as all the same atoms are the same colour. However, the sizes show that chloride atoms are bigger than sodium atoms as can be seen from their arrangement on the Periodic Table. Point this out to learners if you have a Periodic Table stuck up in the class or they can turn to the front of their books to look at the table there.
|
Macroscopic view of sodium chloride |
Submicroscopic view of sodium chloride |
 |
 |
|
Table salt crystals are hard and have a fixed shape. |
Can you see how the chloride atoms (purple) alternate with the sodium atoms (yellow) in a fixed arrangement? |
Take a good look at the picture of the particles in a solid (table salt) above. You will see that they are packed in a regular arrangement. There are very small spaces between the particles in a solid.
Particles are held together by forces of attraction. In solids, these forces are strong enough to hold the particles firmly in position.
Does that mean the particles in a solid do not move at all? No. The particles in a solid move a little bit. They vibrate in their fixed positions. The more energy the particles have, the faster and more strongly they vibrate.
Do you see how we have used the particle model of matter to explain the properties of solids that we can observe? For example, the particles in solids are closely packed and have strong forces between them explains why solids have a fixed shape and you cannot compress them.
An important characteristic of liquids is that they flow. They fill containers they are poured into. Liquids are also not very compressible. How can these properties be explained?

In the liquid state, particles do not have fixed positions. They move about freely, but they stay close together because the forces of attraction between them are quite strong, but not as strong as in solids.
Have you noticed how a liquid always takes the shape of the container it is in? Within the liquid, the particles slip and slide past each other. This is why liquid flows. Their particles are free to move around, filling the spaces left by other particles. Look at the image of the juice being poured. Let's zoom in and have a look at what the particles are doing as the juice is poured.

The particles in a liquid have small spaces between them, but not as small as in solids. The particles in a liquid are loosely arranged which means they do not have a fixed shape like solids, but they rather take the shape of the container they are in.
The speed at which the particles move around inside the liquid depends on the energy of the particles. When we heat a liquid, we are giving the particles more energy and speeding them up.
In gases, the particles move at even greater speeds.
Gases spread out quickly to fill all the space available to them. Think of when you blow up a balloon. The air that you blow into the balloon fills up the whole balloon. A gas will fill the entire space that is available to it. This is because the particles in a gas have no particular arrangement.
Gases do not have a fixed shape. Think about the balloon again: the gas fills the entire space inside the balloon. You can squeeze the balloon, changing the shape.


Gas particles move very fast, much faster than in solids and liquids. The particles in a gas possess a lot of energy.
Have you ever tried to compress the gas in a syringe or in a bicycle pump? Why do you think you can compress the gas?

This is a good demonstration for learners to try. Syringes are cheap and available at most pharmacies. Give each learner three syringes. Let them fill on with sand, one with water and one with air. They then close the nozzle of each syringe tightly with rubber or their finger and squeeze the plunger. Let them observe and try to explain their observations.
In gases, the forces between particles are very weak. This explains why the particles in gases are not neatly arranged. They are not held together tightly and there are large spaces between them. These spaces are much larger than in the solid and liquid state.
Gases can be compressed, because their particles can be forced closer together. Look at the photo of a scuba diver underwater. Do you see the tank on his back? He uses this tank to breathe underwater. A scuba diver can stay underwater for almost an hour. How do you think he can get enough air to breathe for a whole hour from a small tank like that? Discuss this with your class.
The answer is that the air is compressed so that a lot more air fits into the tank than if the air were not compressed. The scuba diver therefore has enough air to last up to an hour.

Let's summarize what we have learned about what the particle model of matter tells us about solids, liquids and gases.
INSTRUCTIONS:
Use the images of the different states to help you, and go back over the text in your workbook.
|
|
Solid  |
Liquid  |
Gas  |
|
Arrangement of particles |
|
|
|
|
Movement of particles |
|
|
|
|
Forces between particles |
|
|
|
|
Spaces between particles |
|
|
|
|
|
Solid  |
Liquid  |
Gas  |
|
Arrangement of particles |
Closely packed in a regular arrangement |
Loosely arranged, but still close together |
No particular arrangement |
|
Movement of particles |
Do not move; only vibrate |
Can move quite fast and slide past each other |
Move very fast |
|
Forces between particles |
Very strong forces between them |
Strong forces, but weaker than in the solid state |
Very, very weak forces between the particles |
|
Spaces between particles |
Very small spaces |
Small spaces |
Very big spaces |
QUESTIONS:
These are extension questions to make sure learners can use what they have learned about the particle model of matter to explain the observable properties of solids, liquids and gases.
Solids have a fixed shape as their particles are arranged in a regular, fixed arrangement and they have strong forces holding them together, so the shape of the solid remains fixed. The particles in a gas do not have any particular arrangement and there are very, very weak forces between them. So, the particles in a gas can easily move around and fill the shape of the container they are in, meaning they have no fixed shape.
The particles in a gas have very large spaces between them, so the particles can be 'squashed' closer together, meaning the gas can easily be compressed to take up a smaller volume. Liquids have very small spaces between the particles and so it is much harder to 'squash' them together, so they are not easily compressed.
The cake flour is not a liquid, but a solid. Flour, and all powders, are solids made of very fine grains which are able to flow freely when they container is tilted or shaken. But these grains are solid.
This is a tricky question and you should discuss it in class. A common misconception among learners is that powders are liquids as you can 'pour' them and they take the shape of the container they are in. They are NOT liquids. Point out to learners that you cannot evaporate a powder, as you can with a liquid, the powder does not make your fingers wet when you touch it.
A video explaining the difference between the solid, liquid and gaseous states of matter.
Have you ever noticed how quickly smells travel? Perhaps you have walked past a rubbish bin, and smelled the garbage.


Have you ever smelled a stink bomb? When you smell these things, how do the 'stink bomb' or 'garbage' particles reach your nose?
Get learners to briefly discuss what stink bombs are for. They may say a stink bomb can be used to play a prank on someone. The smelly particles mix with the air and when we breathe the air, we smell them.
Most smells travel fast, because their particles mix with air and get into our noses when we breathe. We say that the particles diffuse through the air.
In Gr. 7 we learnt about different kinds of mixtures. In the next investigation we are going to explore whether particles mix faster when they are in the liquid state or in the gas state. This is called the rate of diffusion. What would your prediction be?
When we talk about a rate, we are measuring how something changes in relation to another factor, such as time. Another example is speed measured in km/h - this is a rate of how distance in kilometres changes over a period of time (hours).
INVESTIGATIVE QUESTIONS:
At this level, it is sufficient to compare the diffusion rates of liquids and gases qualitatively. We will not perform a controlled quantitative comparison of the diffusion rates. It would be possible to turn the investigation into a controlled experiment, if one used identical containers for comparing the rates of diffusion, and compared gases and liquids that have particles of similar size. It would also be necessary then to choose a gas that is coloured (e.g. bromine gas) so that learners can see the diffusion process inside the container as it progresses. It is important to note that bromine is a hazardous gas and is not freely available. It would only be advisable to use this example if you have the facilities and training to work safely with bromine. An alternative substance which will effectively demonstrate the diffusion of gases is hydrogen sulfide (H2S). A few drops of hydrochloric acid on iron sulfide or sodium sulfide in a conical flask will produce H2S. This can be used instead of the vanilla essence. It is important to note that H2S has a very strong, pungent odour (characteristic rotten egg smell). It is not toxic at low concentrations, but it is important to make sure the room is well ventilated and that the windows are open. It may not be ideal to use H2S as an example if the classroom is very small or crowded. You could also light a 'smoke bomb' (available from toy shops) outside the classroom if this is permitted at your school. The smoke mixing with air is an effective analogy of gases mixing, even though the smoke actually contains fine solid soot particles and is not strictly a gas.
If there is time it is recommended that you repeat the experiment in which gases are mixed (using vanilla essence in a saucer), but on a different day. During the repeat experiments, learners should be allowed to wave the odour particles towards the back of the classroom with their arms. Do this on a different day, to allow the vanilla smell to escape from the classroom, and from learners sensory receptors, between experiments.
HYPOTHESIS:
What are your predictions? Do you expect liquids to mix more quickly than gases, or the other way around? Will stirring influence the speed at which gases mix? Write down your hypothesis below.
IDENTIFY VARIABLES:
This is not a controlled experiment as we are not measuring the rates of mixing of the liquids and gases under exactly the same conditions. We will make a simple comparison of the mixing rates, by seeing how long it takes each to mix under two different sets of conditions.
MATERIALS AND APPARATUS:
METHOD:
Part 1: How fast do liquids mix?
Liquids mix relatively slowly when they are not stirred. It is quite possible that the liquids will not be fully mixed by the end of the lesson, and then learners should note this as an observation. Remind them to check again the following day to see if the colour has spread uniformly through the water.
Part 2: How fast do gases mix?
This experiment should be performed with the windows closed.
Instruct the learners to smell the air and as soon as they can smell the vanilla essence they should quietly put up their hand (without waving it about). Ask the learners beforehand why they should not move while the vanilla essence particles are moving around the classroom. Answer: that would be the same as stirring the mixture, which would make it mix more quickly. It would therefore not be a fair test.
One learner could be tasked with writing the times on the board.
This is an opportunity for learners to see how the mixing time is influenced when they actively mix the air and vanilla essence particles. Get them to predict whether or not the smell will travel faster or more slowly and to discuss possible reasons for this.
RESULTS AND OBSERVATIONS:
Learners should write down what they see. These are possible observations:
How long did it take for the liquids to be fully mixed, until the colour was uniformly spread throughout the water?
If the liquids are not fully mixed by the end of the lesson learners should note this as an observation.
When the air was NOT mixed during the experiment:
Learner/class dependent answers.
When the air WAS mixed during the experiment:
Learner/class dependent answers.
Draw a table with your results for the vanilla essence experiment. You can choose your own column and row headings. Remember to give your table a heading.
An example of the type of table that learners could draw is given below.
Table to show the observations to smell vanilla essence with and without mixing the air.
|
Event |
Time measured without mixing (minutes) |
Time measured with mixing (minutes) |
|
The first learner smelled the vanilla |
|
|
|
Approximately half the class smelled the vanilla |
|
|
|
Learners at the back of the class smelled the vanilla. |
|
|
ANALYSIS AND EVALUATION:
Learner-dependent answer.
Learner-dependent answer.
CONCLUSIONS:
What are your conclusions? (What are your answers to the investigative questions?)
Learners should be able to conclude that gases diffuse more quickly than liquids, and that if you mix or stir the air or liquid, then you speed up the rate of diffusion.
In this investigation we explored the rates at which particles diffuse. What do you think happens at the particle level when two substances mix?
Get learners to discuss this briefly in small groups. Remind them of their observations when the food colouring was mixed with the water. Some ideas to mention are:
An interesting video that explains what diffusion is and how it occurs.
In the photos, we see a yellow liquid being added to a colourless one. Notice how the yellow liquid swirls and spreads out as the yellow particles mix with the colourless particles. Of course we cannot see the particles, but we can make a macroscopic observation (something we can see with the naked eye) of the process.




What will the mixture look like when the coloured particles are uniformly spread out amongst the water molecules?
The mixture will have the same colour throughout. The last photo is almost like this, but not quite.
What will the mixing process look like on particle level? The following diagram represents one of the glasses pictured above, containing a colourless liquid (represented by the blue circles) to which a yellow liquid (represented by the yellow circles) is added. The glass on the left shows the particles in the mixture directly after the yellow liquid was added to the colourless liquid. The glass on the right is empty. You must draw the particles in the mixture after the yellow liquid has spread uniformly throughout the colourless liquid.

Here is what the final drawing should look like. Note that there should be 10 yellow particles in the final container. They should be spread more or less evenly amongst the colourless particles.

When you were watching the coloured liquid mix with the water in the last investigation, was it possible to predict the direction in which the colour would swirl? What made the two liquids mix?
No, it is not possible to predict how the food colouring/coloured liquid will swirl.
The particles in liquids and gases are constantly moving. Their movements are unpredictable: we say the particles move randomly. It is the random movement of the particles that allow liquid and gaseous substances to diffuse.
The following zigzag diagram explains what is meant by 'random' movement. When a gas particle travels from point A to point B, it will collide with many other gas particles along the way - up to eight billion collisions every second! Only a few of those collisions are shown in the diagram. Each time the particle collides, it will change direction. This means the actual distance travelled by the particle is much further than the direct distance between points A and B.
Here we have to be careful not to use words that will leave learners with the impression that the particle has 'will' or moves 'with purpose'. Particles move randomly. If there was just one particle, it might actually follow a random path right out the window! It is because there are so many particles, moving in all directions, that some of them will reach our nose, or the other end of the classroom, over time.

The process responsible for the mixing and spread of particles in a gas and liquid is called diffusion. We can define diffusion as the random movement of liquid or gas particles from a high concentration to a low concentration to spread evenly. The following diagram illustrates the idea in a very simple way: it shows the particles in a gas spreading out over time to fill all the space that is available to it.

The speed at which particles diffuse depends on several factors, namely:
Particles diffuse because they are in constant motion. We found that gas particles diffused much more quickly than the liquid particles in the last investigation. Can we explain that result using the factors listed above?
You can do a practical demonstration of this in class with your learners. Get a group of learners to stand in the middle of an open space. First let them simulate the particles in a liquid, so they should be fairly close together, but still moving around. Then get other learners to move through the crowd of learners in the middle. Get a couple of learners to do this so everyone has a chance. Then get the learners in the middle to simulate the particles in a gas by spreading much further apart and moving around a lot more. They can also bump into each other. The other learners must now also move through the crowd, which should now be much easier and quicker for them to do.
Think of it in this way: imagine you are trying to move through a crowd of people. The closer they are together, the more often you are going to have to change direction to make it through the crowd and the longer it will take to get to your destination.

A particle in a liquid cannot travel very far before colliding with another particle, because the particles are so close together. That means the liquid particles are constantly colliding and are sent into a new direction with each collision. This means the rate of diffusion is much slower in liquids than in gases, because the particles of a gas are further apart and collide much less. Gas particles can travel further without being sent in a different direction by a collision. This is why gases diffuse more quickly.
The following table shows similar zigzag drawings as you saw before, but now you can see the difference between the random movement of a particle through a liquid and through a gas. It will take the particle much longer to travel from A to B in the liquid than in the gas.
|
Liquid |
Gas |
 |
 |
Now that we have a better idea of the behaviour of particles in the different states of matter, we are ready to look at how particles behave when matter changes its state.
In science, a change of state refers to a change in physical state (e.g. when a liquid changes to a solid). What is this process is called?
Freezing
It is always a good idea to learn new things in terms of what we already know. We are going to start this section with a crossword puzzle to revise what we already know about changes of state.
This is an optional revision activity of what learners should have covered in previous grades.
INSTRUCTIONS:

Here are the clues:
Down:
1. If we want to turn steam into water we have to _____ it. (4 letters)
2. The process of turning a liquid into a gas is called _____. (11 letters)
3. The particles of a _____ have large spaces between them. (3 letters)
4. The particles of a _____ are locked in position by strong forces. (5 letters)
5. A solid will change into the liquid state at its _____ point. (7 letters)
7. The liquid state of ice is called _____. (5 letters)
9. The gaseous state of ice is called _____. (5 letters)
11. If we want to turn water into steam we have to _____ it. (4 letters)
Across:
1. The process of turning a gas into a liquid is called _____. (12 letters)
6. The particles of a _____ are close together but they can flow and slide over each other. (6 letters)
8. The boiling point of a liquid is the temperature at which that liquid will start to _____. (4 letters)
10. The solid state of water is called _____. (3 letters)
12. Freezing and melting are the _____ of each other. (7 letters)
13. _____ water turns it into ice. (8 letters)
Here is the completed crossword puzzle.

How can we change matter from one state to another?
Get the learners to come up with some ideas in class. You should eventually guide them to realise that all changes of state involve changes in energy.
Misconceptions about temperature (video).
For matter to change from one state to another, its particles must gain or lose energy. The following diagram shows us that to change the state of a substance, it must either be heated or cooled.
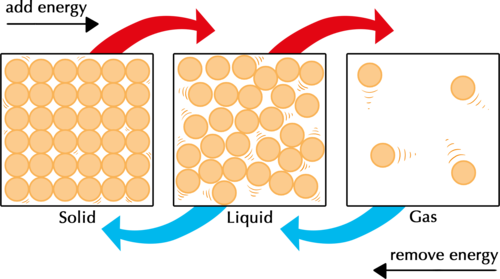
In the figure above, ask learners what they think the little lines around the particles represent. The lines get bigger and further apart as the particles go from solid to liquid to gas. This represent the amount of movement in the particles, as we will discuss in the following content. The kinetic energy of the particles increases as you add energy, and decreases as you remove energy.
First, let us look at what happens to particles when they are heated.
A suggestion is to bring some ice to class and let it melt in a dish for learners to observe. Once the ice has melted, you can then leave the dish out in a hot place so that the next state change can take place and the water evaporates.
When a solid is heated to reach its melting point, it will change into a liquid. This is a process that we are all familiar with, because we have seen how ice melts.

For a solid to change into a liquid state, the particles in the solid need to be freed from their fixed positions in the solid state. How could that occur?
Imagine you are holding hands with a group of other learners. Everyone is jumping in place, much like a solid particle vibrating in a fixed position. The more energetically and randomly everyone jumps, the more difficult it will be for everyone to keep holding hands.
A suggestion is to do this quick and simple demonstration with your learners. Get groups to stand and hold hands. They can start off by swaying and moving their feet. Then they can start jumping. Then they can start jumping higher and swinging and twisting their bodies until they cannot hold each other's hands anymore. These kinds of fun, interactive learning activities make class more engaging and help concepts to stick in learners' minds. When they are standing in one spot but swaying and slightly moving their bodies while holding hands, this represents the solid state. When they are no longer holding hands and are able to move around, this represents the liquid state. Finally, to show evaporation: learners start running/moving around, one at a time. Let them run faster and faster, then break off from the swaying/jumping/moving learners in the central group - this represents a particle that has evaporated and is now in the gaseous state.
When a substance is heated, the particles are given more energy. By giving the vibrating particles in a solid more energy, their vibrations will become more and more vigorous, until the solid particles are able to shake themselves loose from their fixed positions. The forces between the particles are no longer able to hold them together tightly, and the solid melts.
What will happen if we add even more energy to the particles? The particles (which are now in the liquid state) will whizz around faster and faster as they heat up. Soon some of the particles near the surface will have enough energy to escape out of the liquid. Once they are free from the forces that hold them together in the liquid state, they enter the gas (or gaseous) state. The gaseous state is sometimes called the vapour phase, which forms when a liquid evaporates. This is why the gaseous state of water is sometimes called water vapour.
The higher the temperature of the liquid, the faster it will evaporate. A puddle of water will evaporate much faster from the hot pavement than it would from a cool kitchen floor! Why do you think we hang washing outside in the sunshine to dry?
Discuss this with your class. They may simply answer that the sunshine dries the laundry faster, but ask them why they think this happens. It is because the heat from the Sun warms the molecules of the water that is in the wet clothing. When the water molecules are heated, the molecules gain enough energy to escape from their liquid state and the water evaporates. This evaporation will happen much faster outside, in the sunshine, than inside. Encourage them to take down notes as you discuss things in class.

Is there a difference between evaporation and boiling?
Evaporation takes place at all temperatures, while boiling occurs at a specific temperature, called the boiling point. When a liquid is heated to its boiling point, bubbles form in the liquid and rise up to the surface. When this happens, we say the liquid is boiling. Evaporation occurs only on the surface of the liquid, while boiling occurs throughout the entire liquid. Can you remember learning about boiling points in Gr. 7? What is the boiling point of water at sea level?
100°C

Look carefully at the picture of the boiling water above. What do you think is inside the bubbles?
Learners may say that the bubbles are full of gas. Ask them to say which gas. The bubbles are filled with water molecules in the vapour (or gaseous) state. Learners often believe that the water molecules break up into hydrogen gas and oxygen gas when water boils. This is a very common misconception. This section presents an opportunity for learners to recognise that changes of state are physical changes. The molecules (particles) of a substance do not change their composition (that would represent a chemical change) during changes of state. The particles before and after the change of state are exactly the same, they are just in a different state.
Next, we will look at the changes of state that can happen when we cool a substance.
When a gas changes to a liquid, the state change is called condensation. Condensation is the opposite of evaporation. Have you noticed the little droplets of water that form on the outside of a cold glass of water? They are formed by condensation.

When the temperature of a gas is lowered, it takes energy away from the gas particles. The movement of gas particles slows down as their energy decreases and they will start to experience attractive forces. These forces cause them to move closer to each other and they eventually return to the liquid state.
What do groups of people, animals, or birds do when they get cold? They huddle together! In the same way gas particles that are cooled down condense and come together to form water droplets.

What would happen if we cooled the liquid even more? By cooling the liquid, we would be removing energy from it. As the liquid particles lose energy, their movement slows down even more. As their movements become slower and slower, the attractive forces between become stronger. The particles eventually 'lock' into position in the solid state. They can no longer move freely and are only able to vibrate in their fixed positions. We say the liquid has solidified.
The volume of water expands about 9% when it freezes into ice.
This is a relatively short investigation to do in class. Learners should be able to predict what will happen. The skills to focus on here are writing a method for an investigation and recording observations.
This can also be done as a demonstration in front of the class and they must observe the changes of state and record their observations. Learners must write the method themselves. They can either do this in groups and plan how they are going to do the investigation, or if you perform it as a demonstration in front of the class, then they can write the method for themselves afterwards.
You can also use ice in this investigation.
AIM: What is your aim for this investigation?
To observe and record the changes of state when candle wax is heated and when it is cooled.
HYPOTHESIS: What do you propose will happen in this investigation? This is your hypothesis.
A possible hypothesis is: The candle wax will melt when it is heated, and solidify when it is cooled again.
MATERIALS AND APPARATUS:
METHOD:
A possible method which learners might come up with, and which you can follow in class, is as follows: (The steps in a method must be numbered)
Draw a diagram of your setup for the investigation in the following space. Remember to give your diagram a heading and to provide labels.
Learners must draw neat diagrams and label all the equipment used. The label lines must be parallel and drawn with a ruler.
RESULTS AND OBSERVATIONS:
It is a solid at room temperature.
It melted.
It solidified.
The melting point of wax is higher than room temperature as it is a solid at room temperature and needs to be heated in order to melt.
CONCLUSION:
Write a conclusion for this investigation. You must make reference to the particle model of matter in explaining the changes of state that occurred.
A possible conclusion is: When candle wax is heated, energy is added and the particles start to vibrate faster and faster until they break free of their fixed positions in the solid state and enter the liquid state, resulting in the wax melting. When the wax is cooled again, energy is removed and the particles slow down and move slower and slower until the forces between them are strong enough to fix the particles into fixed positions in the solid state and the wax solidifies.
In the next activity we are going to have some fun with water balloons, but not in the usual way. We are going to blow up a balloon without blowing into it and we will make it rain inside the balloon! Sounds like magic? No, just science!
This is an optional activity. You need to be aware of the safety precautions and the fact that learners are working with hot, boiling materials.
For this activity you would need access to a microwave oven. If you do not have one at school or at home, learners could do this activity as a homework assignment if they have microwave ovens at home. An alternative would be to place the balloon in a pot with boiling water for a few minutes.

MATERIALS:
INSTRUCTIONS:
The balloon expands.
It sounds as if it is raining inside the balloon.
The balloon shrinks.
QUESTIONS:
No, the balloon did not have any air inside it because all the air was squeezed out before we started to heat it.
The balloon expanded because the water inside evaporated, filling it with vapour (gas).
What is the name of the gas that made the balloon expand?
Water vapour or steam.
Note: Here is another opportunity to address the misconception that water decomposes into hydrogen and oxygen gas when it boils.
It sounded as if it was raining inside the balloon.
Water droplets falling inside the balloon.
The water vapour condensed inside the balloon to form droplets of liquid water.
It shrank back to its original size.
Evaporation and condensation.
Next, we are going to look at three important properties of matter that are useful to scientists, namely density, mass and volume. These three properties are all related to each other.
You have probably heard the terms mass and volume before in Natural Sciences and Mathematics. But what about density? Have you ever used this word before? Perhaps you have heard someone describe a cake as very dense? What does this mean?
This section introduces us to physical quantities that are important when we study science. Two of these quantities, namely mass and volume, are fundamental properties of matter. We are going to discuss them first, then we will introduce density. Density is another property of matter that is very closely related to the first two.
An interesting video on how to define a kilogram (by using the world's roundest object!)
Look at the picture of a bag of rice. How much rice is in the bag?
1 kg of rice

The mass of an object or a substance tells us how much matter it consists of. The greater the mass of an object, the more matter it contains.
Mass is measured in kilograms (kg). When we measure the mass of small objects or small amounts of matter we often measure in grams (g) or even milligrams (mg).
One kilogram is the same as 1000 grams.
One gram is the same as 1000 milligrams.
How many milligrams are in one kilogram?
Do this calculation on the board. Learners need to be able to interchange between the units. 1000 x 1000 = 1 000 000 milligrams in a kilogram. You can also do some more examples, such as ask how many grams in 1.25 kg (1250 g), how many milligrams in 12.5 grams (12 500 mg)?
If one gold bar has twice the mass of another gold bar, then it contains twice as many gold atoms. The mass of an object stays the same, no matter where it is. Unless a piece of it is cut off, the same gold bar will have the same number of gold atoms whether it is in Gauteng, Bloemfontein, London, or the Moon. That means the mass will always remain constant.
This is fundamentally different to weight, which is dependent on gravity, so the weight of an object will change when we are on Earth or on the Moon. Everyday language confuses the terms mass and weight, especially when talking about body 'weight'.

250 g = 0.25 kg.
The amount of space that an object occupies is called its volume. Volume is measured in litres and is calculated by multiplying the length, width and height of an object. A litre is the space inside a cube that is 10 cm wide, 10 cm long and 10 cm deep.
When calculating volume, 1 cm x 1 cm x 1 cm = 1 cm3. This is the same as 1 ml. That means that 10 cm x 10 cm x 10 cm = 1000 cm3 which equals 1000 ml or 1 litre.

What is the volume of milk in the carton and the volume of juice in the bottle in the following photo?
Milk is 1 litre and juice is 1.5 litres.

When we measure small volumes we use millilitres (ml) as the volume unit. 1000 millilitres is the same as one litre.
Density is a measure of how much mass of a material fits into a given volume. We say density is the ratio of mass to volume. We can write a mathematical relationship to show this ratio as follows: density = mass/volume
We can also use symbols for density (D), mass (m) and volume (V), so the equation to calculate density can be written as D = m/V.
If we have two materials with the same volume, the material with a higher mass will be more dense. It will have a higher density. We can think of density as the 'lightness' or 'heaviness' of objects of the same size.
Think back to the slice of cake that we spoke about as being dense. This is how we can use the word density in everyday language. A piece of cake that is described as dense will feel heavy.

In the next activity we are going to compare different materials that have the same size (or volume), but different densities.
This activity is included now to first introduce the concept of density. We will also look again at the densities of different materials. Learners will have to conduct their own investigation so going through this kind of activity will help them in thinking about the design for the investigation in the section on 'Densities of different materials.'
MATERIALS:
A variety of objects that have the same size (volume) but different densities: sponge, polystyrene, wood, metal, brick or stone.
If you battle to find objects that are the same size, you can start off with some containers that are of equal volume and fill them with different substances. For example, you can use matchboxes (which will all have the same volume), and fill them with different substances such as sand, flour, sugar, cotton wool, etc.
If you do have access to a triple beam balance, do step 3 below and actually measure the mass of each object after arranging them in order of increasing density. This will help to consolidate the relationship between mass and density.
INSTRUCTIONS:
Encourage the learners to discuss the following: Why is sponge so light? Are there any similarities between the way sponge looks, and the way bread looks on the inside? Could this explain why a loaf of bread would be much lighter than a brick of the same size? Learners should be encouraged to notice similarities in the texture of substances of similar density. For instance, bread and sponge have holes or air pockets within the solid material. This means these substances would have less mass per unit volume than materials without holes.
QUESTIONS:
You could bring a brick and a loaf of bread to class so learners can test this out for themselves by handling the two objects.

If they are the same size, it means they have the same volume.
The brick has more mass.
The brick would have the greater density. If we compare two objects of the same size, the one that is heavier (has more mass) has the greater density.
Learn more about density with this simulation http://phet.colorado.edu/en/simulation/density
Here are some tips for teachers for the density simulation http://phet.colorado.edu/files/teachers-guide/density-guide.pdf
We have now learnt about the three states of matter and the properties of each. We know one of the ways in which solids, liquids and gases are different from each other has to do with the distances between the particles in each respective state. The particles in gases are much further apart than the particles in liquids or solids.
Does this mean the different states of matter have different densities? We will find out in the next activity.
This activity is used to explain the general property that solids are more dense than liquids which are more dense than gases. It is mentioned that the boxes contain the 'same material', which is important. Water, is specifically not mentioned in this activity, as it is an exception which will be discussed later. Water does not behave as other materials as the solid phase is actually less dense than the liquid phase in water. Make sure to not refer to water when going through this activity.
INSTRUCTIONS:
They all have the same volume and contain the same material

QUESTIONS:
Container A contains the most particles and C the least.
Note: If learners are unsure, they can do a rough count or estimate of the number of particles in the containers. They must keep in mind that in reality, the number of particles would be impossible to count. It is important that they realise that the density of a gas is significantly lower than the densities of the other two phases.
The container with the most particles would contain the greatest mass; therefore A contains the greatest mass and C the smallest.
The solid has the highest density because it has the greatest mass. The gas has the lowest density because it has the smallest mass in the same volume.
We have just performed a conceptual activity (a 'thinking' activity) in which we compared the densities of the three states of the same material.
Light ice, heavy water! (video)
If possible, play the video 'Light ice, heavy water' by Steve Spangler Science for your learners and ask them why the ice cube floats and the water sinks after watching the video. You can read about the explanation here:http://www.stevespanglerscience.com/experiment/light-ice-cube-heavy-water http://www.stevespanglerscience.com/experiment/light-ice-cube-heavy-water
You could do the demonstration in the video yourself, in class. Do not tell the learners that the 'mystery liquid' is vegetable oil but rather let them try to identify it.
The high density of a solid material explains why it cannot be compressed. The particles in a solid are tightly packed and cannot be squeezed closer together into a smaller volume.
Liquids are also very dense. The density of a liquid is roughly the same as the density of the solid state of the same substance. This is because their particles are close together, even though they are not locked into fixed positions. Most liquids cannot be compressed into smaller volumes.
Liquids are slightly less dense than their solid states but water is an important exception. Have you ever wondered why your ice cubes float on top of the water in your glass?

The solid state of water (ice) is less dense than the liquid, because in ice the water molecules are packed in a unique way. The image below on the left shows shows that water molecules in ice are packed in such a way that there are open spaces between them. On the right, the same water molecules are shown in the liquid state.


Do you see how there are bigger spaces between the water molecules in a solid than in a liquid? This also helps to explain why icebergs are able to float in the sea.

Have you ever seen a frozen bottle of water with the ice pushed up out of the bottle? Why did the water push out of the bottle when it turned to ice?
When the water freezes the particles have larger spaces between them. (Remind them this is a unique and unusual property of ice, that does not extend to all solids.) Once frozen, the same mass of water now occupies more volume. Water (liquid) is more dense than ice. The particles in water are packed closer together. This means more of them will fit into a given volume.
Gases are not very dense at all because of the large spaces between the gas particles. That means they contain a small number of particles in a large volume. This why gases can be compressed: their particles can be squeezed closer together to fit into a smaller volume. Think back to the air that is compressed to fit inside a gas tank for a scuba diver.
In the activity 'Which has the highest density, a solid, a liquid or a gas?'we compared the densities of different states of the same material. This is an easy comparison because the particles in the different states are identical. By comparing the number of particles in the same volume of each state, we can determine the density of each state.
The densities of different materials are slightly more difficult to compare, because different materials consist of particles with differing masses.
The SI unit for density is kg/m3. If you chose to do the density calculations in this section, we will mostly be using g/mL and kg/L as the units of measurement for density as the learners will be working with volumes that they measure in millilitres and litres. These are also accepted units of measurement for density.
We are now going to do a practical activity (a 'doing' activity) to compare the densities of a solid, a liquid and a gas. It would be quite difficult to compare the three states of the same material, as the material would have to be at three different temperatures to be in three different states! For this reason we will compare three different materials: sand, water and air.
INVESTIGATIVE QUESTION:
Which material has the highest density: sand, flour, water or air?
Learners must design this investigation themselves. They can work in groups to do this. They should first discuss how they are going to do the investigation and write down their method in their notebook or on scrap paper. After completing the investigation they should then write up the method in the space provided here.
The list of materials should provide some guidance in terms of a possible procedure. Since density is mass divided by volume, learners could measure the mass of identical cups filled with sand, water and air, and calculate the approximate densities of each material. If you do not have access to a scale, then learners can just compare the densities of each material by holding the cups in their hands.
HYPOTHESIS:
What do you predict: Which material has the highest density: sand, flour, water or air?
IDENTIFY VARIABLES:
If a fixed volume (same size cup) of each material is used, then volume is the constant or fixed variable. The cups must all be made of the same material so that they have the same masses.
The independent variable is the type of material.
Mass is measured and used to calculate density.
MATERIALS AND APPARATUS:
METHOD:
You will be designing this investigation yourself. If you are working in groups, you need to first discuss how you are going to conduct (carry out) this investigation. This is the planning. Write down your proposed method in your notebook or on scrap paper. Discuss this with your teacher. Remember to also think about how you are going to record your results. After you have conducted the investigation, write down your method on the lines provided here. Summarise each step in sequence and number the steps.
Learners must write the steps for their investigation in a numbered sequence. If you have access to a scale or triple beam balance, then they must measure the mass of each cup and use this to calculate the density. They will need to know the volume of the cups to do this. The volume might be written on the cups, but if not, ask them how they are going to determine the volume. A suggestion is to fill the cup with water, then pour this water into a container which has measurement (such as a beaker or measuring cylinder) and then record what the volume is.
RESULTS AND OBSERVATIONS:
What were the results of your investigation? Summarise them below. You can draw a table. If you were able to measure the mass of each cup, show your calculations for the density of each material.
If learners were able to measure the masses of the cups containing different materials, then they must calculate the densities using the equation\(D = m/V\).
An example of a calculation might look like this:
Mass of cup of flour = \(\text{150}\) g
Volume of cup = \(\text{250}\) mL
\(D = m/V\)
= 150/250
= \(\text{0,6}\) g/mL
ANALYSIS AND EVALUATION:
Learner-dependent answer.
Learner-dependent answer.
Learner-dependent answer. They should include something about using the same cup for each measurement.
CONCLUSION:
What is your conclusion? (What is your answer to the investigative question?)
This investigation should show that equal volumes of these different materials have different masses and therefore different densities. You can also point out that they also compared materials in different states, and they also compared two solids, namely sand and flour.
In the last investigation we saw that two solids, namely sand and flour have different densities as they are different materials. But what about liquids? Do all liquids have the same density or does the type of material of the liquid affect the density?
Have you ever noticed that oil floats on water?

When you mix oil and water, as in the picture of the salad dressing below, the two materials will eventually separate because they do not mix well. They are immiscible. When they separate, the oil will always float on top. The two separate layers of water and oil are referred to as 'phases', the oil phase and the water phase.
Create your own fluid simulation of oil and water. http://www.escapemotions.com/experiments/fluid_water_3/
Bring cooking oil to class and demonstrate this by pouring some oil into a glass of water. Stir it to 'mix' the oil and the water and then allow them to separate out again into the different layers. The simulation link given in the visit box is quite fun for learners to experiment with and see what happens on a particle level when you mix oil, water and foam, and they can watch them separate out again.

Oil floats on water for two reasons:

Oil does not dissolve in water. The oil molecules cluster together and float on the surface. If a large amount of oil is poured into water, the oil will spread out and form a layer on the surface of the water. Oil that is spilled into the ocean or a lake spreads over a huge area. It poisons many animals, birds, fish and plants and is very expensive to clean up. That is why oil pollution has an extremely negative impact on our environment.


When two substances are in the same container, but not mixed (like oil and water for instance), they will form two layers. In a certain sense, water and ice also form two 'layers'. Which layer will be on top: the one which is more dense or the one which is less dense?
The layer which is less dense will float on top of the layer that is more dense.
In the next activity we look at how we can layer different liquids on top of each other depending on the densities!
This activity can be done as a fun demonstration for of the class. It gives a very clear illustration of the differences in density of different liquids. You do not have to use all of the items in the list of materials given below, as long as you have a few of different densities. Watch this Steve Spangler Science video in the visit box before doing this demonstration in class to get a good idea of how to demonstrate it correctly.
A video showing how to make a rainbow density column.
MATERIALS:
INSTRUCTIONS:
If you have equal volumes of each liquid, the mass and density will be related and the heaviest liquids will be the most dense. Draw a table on the board to record the masses of each liquid.
If you are using the suggested liquids in the list given, the order to pour them in is as follows: honey, golden syrup, milk, dish washing liquid, water, vegetable oil, rubbing alcohol.
QUESTIONS:
The honey is the most dense as it is at the bottom; the rubbing alcohol is the least dense as it floats on top of the other layers.
Yes, there is a relationship. In equal volumes of the liquids, the liquid that is the heaviest is the most dense.
Note: This introduces the idea of equations to explain scientific phenomena (density = mass/volume). If the volume remains the same and the mass increases, then the density must also increase. Learners do not need to do calculations at this level, but if you would like to extend the exercise, you could work out the densities of each liquid using the measured mass and the volume for each liquid.
Dependent on objects used. If the suggested ones are used, the order would be: bolt, popcorn kernel, cherry tomato, beads, ping pong ball. The most dense objects will be at the bottom and the least dense at the top.
The objects drop to the different levels depending on their densities. The metal bolt is more dense than any of the liquids so it sinks to the bottom. The other objects will sink to the layer at which their density is equal to that of the liquid.
Note: Learners might battle with this, but you can give them an example. For example, the plastic beads are less dense than water and the liquids below that, but they are more dense than the vegetable oil and the liquids above that. So, the beads will float on top of the water layer.
Depending on the objects used, but from the suggested list, those less dense than water are the ping pong ball and plastic beads, those more dense than water are the cherry tomato, popcorn kernels and bolt.
This is an optional extension activity if you would like to do some density calculations. Calculations will become an important part of physical sciences in Gr. 10-12 and so it is helpful if learners start using some of the more simple equations now.
INSTRUCTIONS:
|
Material |
density (g/mL) |
|
water (liquid) |
1 |
|
ice |
0.917 |
|
glass |
2.6 |
|
salt |
2.2 |
|
chalk |
2.36 |
|
coal |
1.5 |
|
cork |
0.25 |
QUESTIONS:
\(D = m/V\)
= 500/555
= 0.9 g/mL
Chalk is more dense.
Density of glass = 2.6 g/mL.
\(D = m/V\)
\(V = m/D\)
= 50/2.6
= 19.2 mL
The coal will have the greater mass as it has a higher density.
Mass of coal:
\(m = DV\)
= 1.5 x 100
= 150 g
Mass of cork:
\(m = DV\)
= 0.25 x 100
= 25 g.
We have learnt that the density of a material depends on how tightly packed the particles inside the material are. The more tightly packed they are, the more dense we say they are.
The following diagram represents a container (on the left) that contains a small amount of gas. Imagine that all the gas from the small container is moved into the empty container on the right. Draw the gas particles in the container on the right.

The learner's sketch should show 10 particles, spread out evenly to fill the larger container. Note that the particles should have the same size as before.

A gas will expand to fill whatever space it is in. In the larger container we will still have the same number of gas particles, but now they are filling a much larger space.
If we take a certain amount of gas from one container and place it into another, larger container, the gas expandsto fill the larger container. The same mass of gas is now in a larger volume, the gas now has a lower density.
Solids and liquids cannot behave in this way. Their densities will remain more or less constant no matter in which container they are placed. This is because their particles are relatively close together with strong forces between them. But what happens when we heat them? We have learnt that this is the same as giving them extra energy. How will heating them affect the packing of the particles and the density?
Get learners to discuss this question. They have learnt that particles move faster at higher temperatures. How would this affect the spaces between particles? Most solids and liquids tend to become less dense as they warm up. The learners do not need to come to any conclusions at this point. The question will, however, help to introduce the concepts of contraction and expansion.
In the next section we are going to look more closely at what happens to the particles inside materials when they expand. We are also going to look at the opposite of expansion, namely contraction.
Have you ever been inside a tin-roofed house? On a hot days, you often hear the metal roof panels groan and creak. Do you know why this happens?

Some materials become slightly larger when they are heated. We say they expand. Materials can also shrink slightly when they are cooled. We say they contract.
The metal roof panels expand and contract as the outside temperature changes. When this happens, the panels scrape against each other and against the nails that keep them in place. The scraping of metal against metal causes the creaky, groaning noises.
How is it possible for materials to contract and expand? Can you think of an explanation?
Get learners to question the possibility of new atoms forming inside the material as an explanation for the phenomenon of expansion. Guide them to the law of conservation of mass: matter cannot be created or destroyed. Materials expand and contract due to particles moving further apart or closer together, not because the number of particles increases or decreases.
To understand this phenomenon, we will look at some examples of expansion. We will then try to explain expansion in terms of the particle model.
Some solids expand more than others. When we choose materials for a new job, it is important to know how much they will expand. This way we can allow for expansion when the materials get hot.
In the following diagram, the picture on the left shows a concrete path or road surface. How have the engineers who built the road allowed for expansion?
The road was built in segments, with small gaps between segments to allow for expansion.

The picture on the right shows what could happen if no allowance is made for the expansion of the concrete blocks. The forces created by the expansion of the concrete are so strong that the road surface has cracked!
This is a very important principle to remember when building bridges. When engineers design a bridge, they must allow for contraction and expansion of the materials used to build the bridge. Have a look at the following photo showing a close-up of the gap between the two road surfaces of a bridge. Can you see the interlocking 'teeth'? These allow the bridge to expand and contract while the teeth slide past each other.

In this activity we will compare the expansion of different solid materials by drawing a graph. You will need the following information for your graph:
|
Material |
How far a 100 metre length of the material will expand when the temperature increases by 10°C |
|
Brass |
19 mm |
|
Iron |
12 mm |
|
Steel |
11 mm |
|
Platinum alloy |
10 mm |
|
Concrete |
11 mm |
|
Ordinary glass |
11 mm |
|
Ovenproof glass |
3,5 mm |
Draw a bar graph with 'Expansion' on the y-axis and 'Materials' as categories on the x-axis. Choose an appropriate title for you graph.
Here is the bar graph that learners must draw. Any appropriate title will do, for instance: Expansion of different materials when temperature is increased by 10°C (per 100 meters of material).

Q UESTIONS:
Brass
Ovenproof glass
Which solid would be the best material to reinforce concrete? (Hint: the reinforcing material should expand as much as the concrete, otherwise it will damage the concrete during expansion.)
Steel. It expands the same amount as concrete.
From the graph and the data in the table, we can see that brass expands much more than ordinary glass. When the weather is really hot, the brass expands so much that the window glass does not fit properly anymore and falls out. You could advise the owner to try the following:
Note: In addition to the type of material, the amount of expansion also depends on how much material there is. This is why expansion is difficult to see in relatively small items. e.g. cooking pots. A key will still fit in a lock, even if the key has been lying in the hot sun, because expansion is not that noticeable in small items.
The following diagram shows a metal ball and ring apparatus. The ring and ball are both made of brass. At room temperature, the ball is just the right size to pass through the ring.

Do you think the ball will still fit through the ring when the ball has been heated?
The ball does not fit through the ring when the ball has been heated.
Note: This is a great way to demonstrate contraction and expansion if you have one of these at your school.
If you are doing a practical demonstration, get the learners to make a prediction before continuing with the demonstration. You can heat the ball in the flame of a Bunsen burner.
No, the brass ball cannot have a greater mass because it has not gained any mass.
Note: We know this because matter cannot be created (or destroyed).
The ball will contract to the same size as before because it has not gained or lost any mass.
Note: If doing the practical demonstration, get the learners to make a prediction in the time that it takes for the ball to cool down.
Now that we have seen that materials can expand, how can we explain expansion of a material in terms of the behaviour of the particles in that material?
Get learners to discuss this for a few minutes. Guide the discussion with the following questions: What are we adding to matter when we heat it? (Energy.) What do particles do when they are given more energy? (They move faster.) What happens to the spaces between the particles when they start to move faster and bump against each other with more force? (The spaces get bigger as the particles start moving apart.) Learners should be encouraged to take notes in discussions.
We have learnt that when matter is heated, the particles of that matter will move faster and push further apart from each other. What happens to the particles in matter when it is cooled?
Get learners to discuss this for a few minutes. Guide the discussion with the following questions: What are we taking away from matter when we cool it? (Energy.) What happens to particles when they lose energy? (They move more slowly.) What happens to the spaces between the particles when they start to move more slowly? (The spaces get smaller as the particles start moving closer together.)
When a substance cools (energy is removed), the particles in that substance will slow down and move closer together. That is why most materials contract when they are cooled.
Let's look at a thermometer to understand expansion and contraction.
In Gr. 7 Matter and Materials, learners were introduced to the bulb thermometer and the idea that materials expand and contract due to changes in kinetic energy of the particles (the size and number of particles remains the same, only the spaces between the particles increase or decrease). If you have access to a Gr. 7 workbook, you can look at what they did. You can also download the PDFs of these books from the website and view the content online. In this activity these ideas are revisited and expanded upon, with the particle model as frame. Important links are established between expansion and contraction and their effects on the properties volume and density.
The common glass thermometer is called a bulb thermometer. All bulb thermometers have a fairly large bulb that is connected to a long, thin tube. The thermometer has a brightly coloured liquid on the inside. Some thermometers contain mercury as it expands and contracts quite a lot when heated or cooled.
Mercury is the only metal that is a liquid at room temperature.
Look carefully at the following set of diagrams. They represent the same thermometer at two different temperatures.

QUESTIONS:
20°C
The temperature reading on the thermometer on the right is 10°C.
Circle A is the correct one. The lower temperature has made the liquid inside the thermometer contract, so the particles are closer together.
Note: This question provides an opportunity to identify learner misconceptions about expansion and contraction:
No, the material cannot have less mass because it still has the same number of atoms as before and their mass is constant.
Note: This is a good time to remind learners that matter cannot be created or destroyed.
Answer B would now represent the thermometer at a higher temperature of 30°C as the particles have gained more energy and therefore moving at greater speeds so they move further apart and the liquid expands.
The volume of the material increases, because the particles move further apart. The material expands.
The mass does not change but the volume increases, therefore the density must decrease.
Note: It may help to refer back to the formula: density = mass/volume.
When a material is heated, its particles move further apart. When the material cools down, the particles move closer again. Heating and cooling cause the volume of the material to change.
We have learnt that thinking about matter in terms of the particles inside it can help us to understand many interesting phenomena: the physical properties of the different states of matter, changes from one state of matter to another, density, and expansion and contraction.
How can we measure how much of a liquid or a solid we have? If we want to know how much of a material we have, we can measure its mass. What instrument do we use to measure mass?
A scale or a balance.

Think back to the investigation comparing the densities of sand, water, flour and air. How did you measure the mass of the air in a cup?
Most likely, the learners were unable to measure the mass of the air. If they managed to do so, it would probably have required quite a complicated process. They should be reminded that the air is not without mass, but that the mass of the air inside the cup is so small. (It is actually not possible to measure the mass of air when it is surrounded by more air. One way to measure the weight of air would be to do so in a vacuum. But this is beyond the scope of this discussion here for the learners.)The purpose of this short discussion is to prepare learners for the section about pressure. Measuring the pressure of a gas is one way to 'measure' how much gas we have.
We are now going to shift our focus to gases. Gases have much lower densities compared to solids and liquids. That means a large volume of gas will have a small mass. Small masses can be difficult to measure without a special, super-sensitive scale. Scientists have devised a different way of measuring how much of a gas they have.
We have learnt that gases contain millions of fast-moving particles. The following picture represents gas particles inside a container.

As the particles whizz around, they bump and bounce off each other. They also bump against the inside of the container. The force of the particles bumping against the sides of the container cause a phenomenon called gas pressure. The number of bumps (or collisions) will depend on the number of gas particles in the container. More particles inside the container means more collisions, and more collisions mean a higher pressure.
If we can measure the pressure of the gas, we will have an idea of how much gas is inside the container.
Wind is simply moving air! The movement of the air is caused by differences in pressure between one area of the Earth's atmosphere and another. When the wind blows, it is the atmosphere equaling out uneven pressures by moving air from a high pressure area to a low pressure area.
Have you ever seen anyone check the pressure in a car tyre? You may have seen them use a device like those in the photo below. It is called a tyre pressure gauge and it is specially designed to measure the air pressure inside a tyre.

The round end of the gauge should be pressed against the air valve of the tyre. This opens the valve and lets some of the air from the tyre escape into the gauge. The air particles bump against a disc inside the gauge. The force generated by many gas molecule collisions pushes out a bar at the back of the gauge. Can you see it in the picture? For this particular pressure gauge, the pressure inside the tyre is indicated by how far back the bar is pushed out of the back of the gauge. Note the numbers along the bar which allow us to measure the pressure.
How to check tyre air pressure: http://www.howstuffworks.com/pressure-gauge.htm
Other, more complicated pressure gauges all work in a similar way.


How could we increase or reduce the amount of gas in a container? In the next activity we are going to see if we can understand gas pressure in terms of the particle model of matter.

One of the learners could bring their bicycle and pump to class to demonstrate the final activity.
MATERIALS:
INSTRUCTIONS:
It is easier to blow into the bag when it is 'empty', or when there is not much air in it and becomes increasingly more difficult to blow into as it fills with air.
Note: If the bag is strong it may not be possible to pop it by blowing into it, because humans cannot blow with much force. A 'weak' bag may come apart at the seams or start to leak when the learners continue to force air into it beyond its capacity. Ask them to think about why this happens.
This step requires a balloon.
The balloon is difficult to blow into at first. Thereafter it becomes more easy to blow air into the balloon. When the balloon becomes stretched 'to capacity' with air, it becomes more and more difficult to blow more air into it.
Note: The balloon is difficult to blow into at first because the material (rubber) that the balloon is made of is still very tight. It can be relaxed somewhat by stretching the balloon before blowing into it, but the first one or two 'blows' will require some effort.
Tie a knot in the top of an inflated balloon. Leave the balloon in the classroom and examine it again after one week. Does it look the same as when you inflated it a week ago? Perhaps it looks a bit like this balloon in the following photo:

Note: After one week the balloon has become wrinkled and it is smaller than it was originally.
This step requires a balloon and an empty plastic bottle.
It is impossible to blow up the balloon.
The balloon swells up and fills the inside of the bottle.
This step requires a bicycle tyre and pump.
At first it is easy to pump air into the tyre but, the more you pump, the more difficult it becomes.
QUESTIONS:
Try to answer the following questions by explaining what is happening to the air particles in each case. Use the words 'particles', 'collisions' and 'pressure' in your answers.
Air particles are forced into the bag, balloon or tyre. The more you blow, the more particles are pushed into the bag, balloon or tyre. If there are more particles, there will be more collisions, and that means the pressure will be greater.
When the bag contains as many particles as it possibly can and we try to force even more particles into the bag, the force of the collisions of the particles against the inside of the bag becomes too much and the bag breaks.
When the balloon contains as many particles as it possibly can, it will be stretched to its maximum size. If we try to force even more particles into the balloon, the force of the collisions of the particles against the inside of the balloon becomes too much and the balloon pops.
Note: Actually the polymer strands of the balloon tear apart as a result of the applied force.
Why do you think the balloon became smaller when it was left for a week? The following diagram should provide a hint:

Air particles escaped through tiny openings in the material of the balloon. There are now fewer particles inside the balloon so there are fewer collisions against the inside. The force pushing against the inside of the balloon is smaller, therefore the pressure inside the balloon is smaller.
Note: Learners may answer that the air inside the balloon has contracted and while this may be part of the explanation, there is another factor that contributes much more significantly to the shrinkage over time: air particles will slowly escape from the balloon over a period of time because the balloon polymer has tiny holes. These holes are much too small to see with the naked eye, but they are large enough for the air molecules to escape through.
The balloon does not inflate much because the bottle is already filled with air. There is no room for the balloon to expand inside the bottle. The air particles inside the bottle push back against the air particles inside the balloon. When the bottle has a hole in it, the air particles inside the bottle can be pushed out through the hole, as the balloon fills the space inside the bottle.
The more you pump, the more particles are pushed into the tyre. If there are more particles, there will be more collisions, and that means the pressure will be greater. The tyre will stretch to accommodate more air particles, but it will become more and more difficult (require more and more force) to stretch the tyre material.
The following two paragraphs are not required by CAPS but may be included as enrichment. This introduces some concepts to be dealt with in the later grades.
If the gas is heated, the particles will move faster as they gain more energy. That means they will collide with the inside of the container more often and with more force. This causes an increase in pressure.
If the gas is cooled, the particles will move more slowly, because they will have less energy. The gas pressure will decrease, because the particles will bounce against the inside of the container less frequently and with less force. Look at the following table which illustrates this.
|
Cool gas |
Hot gas |
 |
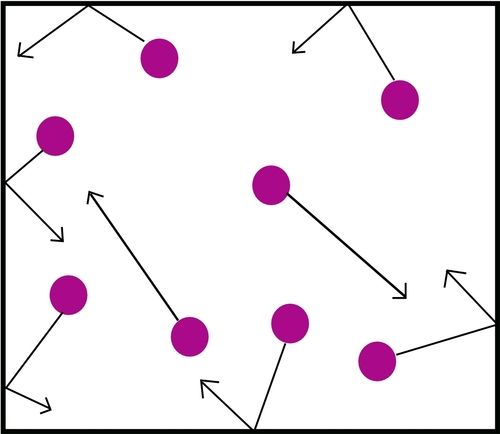 |
|
Fewer and less energetic collisions. |
More and more energetic collisions. |
When a gas is squeezed into a smaller volume, the particles have less space to move. This is shown in the diagram below. Have you noticed that when people are squashed into small spaces, they bump into things more often? In the same way, the gas particles will collide more often with each other and with the inside of the container if they have less space to move in. More collisions means increased pressure!

We have learnt that a gas will expand to fill all the available space. So, what will happen if we take a certain amount of gas out of one container and place it into another container that is twice as large?
The gas will expand, filling the larger container.
We still have the same number of gas particles, but now they are inside a much larger volume. There is twice as much space between the molecules as there was in the smaller container.
What has happened to the density of the gas? Has it increased, decreased or stayed the same?
The density has decreased because the volume increased.
In this chapter, we learnt how many different physical properties of matter can be better understood when we think in terms of the behaviour of the particles in the matter.
The particle model describes the particles in solids as follows:
The particle model describes the particles in liquids as follows:
The particle model describes the particles in gases as follows:
Changes of state are usually the result of heating or cooling:
The density of a material depends on two things:
Concept map
Have a look at the concept map that shows how the many concepts relating to the particle model of matter fit together. There are 4 empty blocks which you need to fill in.

Teacher's version

Use this question to see what learners understand by the model. They should mention something such as: The particle model of matter is a scientific theory which is used to explain how all matter (solids, liquids and gases) are made up of particles and how they behave in the different states. Learners could also mention something about the size of the particles, and that there are empty spaces between the particles.
Water is unusual in that the solid state is actually less dense than the liquid state and so ice floats on liquid water. This is because there are large spaces between the particles in the solid state, making the ice less dense.
Complete the following table with the terms and definitions of different changes of state. [4 marks]
|
Change of state |
Explanation |
|
|
When heat is added and a solid changes to a liquid |
|
Condensing |
|
|
|
When heat is added and the particles at the surface of a liquid change to the gas state |
|
Solidifying |
|
|
Change of state |
Explanation |
|
Melting |
When heat is added and a solid changes to a liquid |
|
Condensing |
When heat is removed and a gas changes state to a liquid |
|
Evaporating |
When heat is added and the particles at the surface of a liquid change to the gas state |
|
Solidifying |
When heat is removed and a liquid changes to a solid |
When heat is added, the particles start to vibrate faster and faster (as they gain kinetic energy), until they move fast enough to overcome the forces holding them together in fixed positions in the solid. The particles are then able to move and slide past each other as they are not held in an orderly arrangement anymore in the solid, and the solid becomes a liquid.
Complete the following sentence by writing it out in full again: During expansion, the spaces between the particles get _____, and during contraction, the spaces between the particles get _____. [2 marks]
During expansion, the spaces between the particles get bigger, and during contraction, the spaces between the particles get smaller.
When the metal expands (because it is heated) the particles will move further apart. The piece of metal will get bigger, but it will still have the same number of particles and so it will still have the same mass. The density of the metal has decreased.
Oil is less dense than water so it floats on top of water.
Learner's picture should show random movement of the perfume particle, with many changes of direction.
The petrol fumes around the petrol station are in the gas (or vapour) state. The particles in the gas can move freely and diffuse into the air around the pumps. If you light a match close to the pumps, the petrol gas can be ignited and this can cause a fire or explosion.
Note: Usually the underground fuel tanks are safeguarded against explosions and fires. Some people say that small sparks (caused by static charges) from cell phones can also cause ignition of the petrol fumes, but scientists generally do not agree.
Air is a gas and water is a liquid. Gas particles have large spaces between them and that means they are easy to compress, or squeeze together. The spaces between liquid particles are so small that liquids cannot be compressed. That is why the plunger moves when there is air in the bicycle pump, but does not move much when the pump contains water.
The following table represents a summary of the entire chapter. You must complete it, using your own words and or diagrams. Some of the blocks in the table already contain information to help you form your own sentences. [18 marks]
|
State of matter |
Solid |
Liquid |
Gas |
|
Diagram showing how the particles are arranged |
|
 |
|
|
Arrangement of the particles |
Very closely packed. Regular arrangement |
|
|
|
Spaces between particles |
|
|
Very large |
|
Forces of attraction between particles |
|
Strong, but weaker than in solids |
|
|
Movement of particles |
|
|
Fast and random movement |
|
Shape |
|
No fixed shape Depends on the container |
|
|
Volume |
|
|
No fixed volume Depends on the container |
|
Compressibility |
Cannot be compressed |
|
|
|
Diffusion |
|
Diffuses slowly |
|
|
Density compared to the other states |
Highest density (except in the case of ice) |
Almost as dense as the solid |
|
|
State of matter |
Solid |
Liquid |
Gas |
|
Diagram showing how the particles are arranged |
 |
 |
 |
|
Arrangement of the particles |
Very closely packed. Regular arrangement |
Close together, but disordered. |
Far apart and disordered. |
|
Spaces between particles |
Very small spaces. |
Small spaces. |
Very large |
|
Forces of attraction between particles |
Strong |
Strong but weaker than in solids |
Very weak |
|
Movement of particles |
Vibration only |
Sliding movement, random. |
Fast and random movement |
|
Shape |
Fixed shape |
No fixed shape Depends on the container |
No fixed shape |
|
Volume |
Fixed volume |
Fixed volume |
No fixed volume Depends on the container |
|
Compressibility |
Cannot be compressed |
Slight compression is sometimes possible |
Very compressible |
|
Diffusion |
Does not diffuse |
Diffuses slowly |
Diffuses quickly |
|
Density compared to the other states |
Highest density (except in the case of ice) |
Almost as dense as the solid |
Lowest density |
Total [42 marks]
|
Previous
Chapter 5: Atoms
|
Table of Contents |
Next
Chapter 7: Chemical reactions
|